The blue curve is the plot of the data. The orange line is tangent
to the blue curve at t = 40 s.
The blue curve is the plot of the data. The orange line is tangent
to the blue curve at t = 40 s. 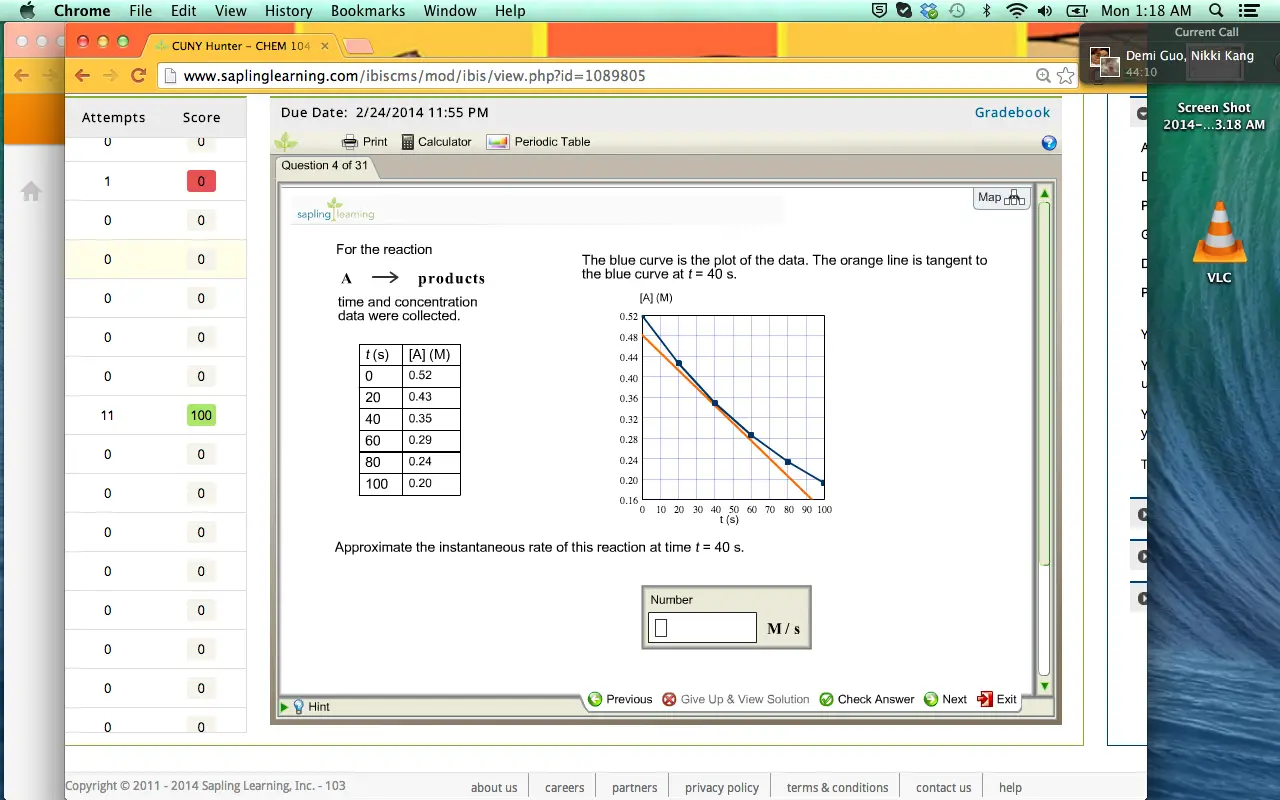 The blue curve is the plot of the data. The orange line is tangent to the blue curve at t = 40 s.
The blue curve is the plot of the data. The orange line is tangent to the blue curve at t = 40 s.
General guidance
Concepts and reason
This problem is based on the concept of chemical kinetics.
Kinetics tells us that how fast or slow does a chemical reaction take place.
Instantaneous rate of reaction is calculated by the graph between reactant/product concentration and time.
Fundamentals
The rate of a reaction is the rate of change of concentration of reactants or products with respect to time.
For a reaction as follows:
Here, A and B are reactant and product respectively.
The rate of a reaction is mathematically expressed as follow:
Here, r is the rate of reaction, is the rate of change of concentration of reactants and dt is the change of time.
The slope of a line is as follows:
Here, m is the slope of the line, and are two y coordinates, and are two x coordinates on the line.
Step-by-step
Step 1 of 2
To determine instantaneous rate of reaction at time equal to 40 seconds, a tangent is drawn at this point. The orange line shown below is the required tangent at .
Here the slope of the tangent at gives out the instantaneous rate of reaction at this point.
Calculate the instantaneous rate of reaction at
Step 2 of 2
Instantaneous Rate is equal to the slope of the tangent line at
Substitute 0.48 M for, 0.16 M for, 0 s for and 92 s for in above equation as follows:
The approximate instantaneous rate of reaction at is .
The instantaneous rate is the rate at a point in the plot at a particular time. Instantaneous rate of reaction is calculated by the graph between reactant/product concentration and time.
The formula for slope can be wrongly taken as follows:
But the correct formula is as follows:
Answer
The approximate instantaneous rate of reaction at is .
Answer only
The approximate instantaneous rate of reaction at is .
A →B
d[4] r=- dt
xx – –
t = 40 s
[A] (M) 0.52 0.48 0.44 0.40 0.36 0.32 0.28 0 24 0.20 0.164 0 10 20 30 40 50 60 70 80 90 100 t(s)
t = 40 s
t = 40 s.
t = 40 s.
xx – –
) (0.48 M -0.16 M Os-92 s = 3.478×10- M/s
=%+} +
xx – –
t=40s
3.478×10- M/s
t=40s
3.478×10- M/s
We were unable to transcribe this image
- What Does Ominous Spiritus Mean? - March 25, 2024
- Who is hat mccullough? And was he an actualy serial killer? - March 25, 2024
- how do i find the 2 kids 1 sandbox video? i really want to see it.? - March 25, 2024
