6. & 7.
please explain/show steps

Activity B (continued from previous page) Hy sou +2 NOCH N-2S0y +2H20 4. Calculate: Select the Worksheet tab. This tab helps you calculate the analyte concentration • Fill in the first set of boxes (“moles H,SO,” and “moles NaOH”) based on the coefficients in the balanced equation. If there is no coefficient, the value is 1.) Record the appropriate volumes in the “ml NaOH” and “ml. H SO,” boxes Record the concentration of the titrant in the M NaOH box Click Calculate. What is the concentration listed? Imol H2SO.21.bml NaOH. IM NACH.108 2met NACH To ML #2504 Click Chock. Is this the correct concentration?_10% If you get an error message, revise your work until you get a correct value. (You may have to redo the titration if you do not have the correct volume of titrant.) 5. Practice Perform the following litrations and determine the concentrations of the following solutions. In each experiment, Ist the volume of bitrant needed to neutralize the analyte and the indicator used. Use the Worksheet tab of the Gurmo to calculate each analyto concentration. Include all units. Titrant Analyto Indicator Titrant volume Analyte concentration HBO 0.70 M KOH 0.50 M HCI Methyl orange 30mL Ca(OH), Phenolphthalein 8.4mL Bromthy med blue 5.6ml 210M 1.02IM 1.090M 0.80 M H, SO NaOH 6. Anny: Once you know the concentration of a strong acid or a strong base, you can estimate its pH. Use ph-logo1H”) to calculate the pH of each of the strong acid mystery solutions (Mystery HBr and Mystery H2SO4) based on the concentrations you determined in questions 4 and 5. Check your answers with the Gizmo. (Because dissociation is not always complete your answers may vary slightly from values in the Gizmo.) [H,801 – pHH,30, [HB- pH HB 7. Apply for a strong base, the concentration of hydroxide ions (OH)in roughly estimated to be the same as the concentration of the base. The pH of a strong base is found with the equation pH = 14 + logo(OH). Based on their concentrations, find the pH of each of the strong bases. Check your answers with the Gizmo Ca(OH) – pH Ca(OH), INAOH p14 MIMO |
pH = – Log [ H+ ]
pOH = – Log [ OH- ]
pH = 14 – pOHplz
like
Cobus ( [caconse) = 0.021m Со ои), Co°4 + 2 бит (би” ) 2 2 ур бэ. 1 т. 2 орчә. (1) pon Сиотво – Gehoooj Boeke 9 E-, а ба – 2 had ahb – 1 – 1,2, Nat А он 12. (, ) TpH = 12.62). fon Cacord дори о со м Јаби (би”) : о со м уби – – og Coи” ) + on Cod96) 1 «Ч, – 40 400whI 2 10: – 1 2 си = •9S й (Мари) = av95
We were unable to transcribe this image
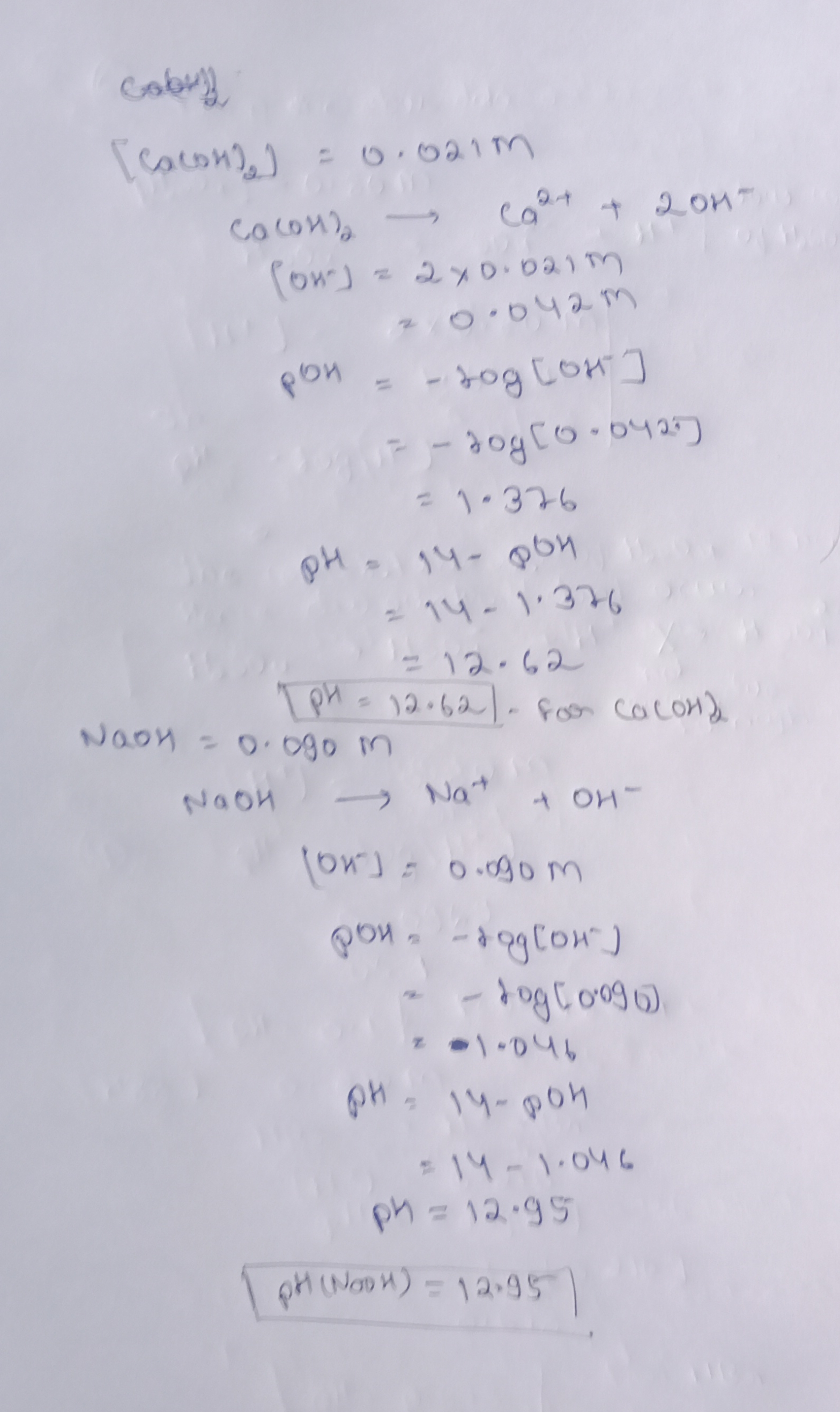

- Looking to Rank on Top? Trust SEO Experts in New York to Get You There - September 30, 2024
- How to fix a configuration registry database corrupt from command prompt from a fresh windows repair tool - September 30, 2024
- camera in use by another app - September 30, 2024
