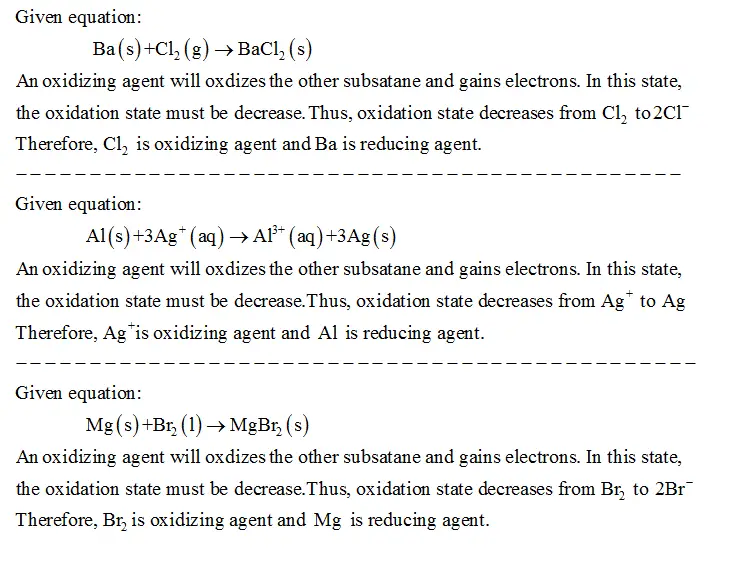
1)Establish the oxidizing agent and the lowering agent for
Ba(s)+Cl2(g)→BaCl2(s) . Categorical your solutions as chemical formulation separated by a
comma. oxidizing agent, lowering agent_______. 2)Establish the oxidizing agent and the lowering agent for
Al(s)+3Ag+(aq)→Al3+(aq)+3Ag(s) . Categorical your solutions as chemical formulation separated by a
comma oxidizing agent, lowering agent_______. 3) Establish the oxidizing agent and the lowering agent for
Mg(s)+Br2(l)→MgBr2(s) . Categorical your solutions as chemical formulation separated by a
comma oxidizing agent, lowering agent_______.
Given equation: Ba(s)+C12 (g) → Baci, (s) An oxidizing agent will oxdizes the opposite subsatane and features electrons. On this state, the oxidation state should be lower. Thus, oxidation state decreases from Cl2 to2cI Due to this fact, Cl2 is oxidizing agent and Ba is lowering agent. Given equation: AI (s)+3Ag (aq-Al(aq)+3Ag(s) An oxidizing agent will oxdizes the opposite subsatane and features electrons. On this state, the oxidation state should be lower.Thus, oxidation state decreases from Ag to Ag Due to this fact, Agis oxidizing agent and Al is lowering agent. Given equation: Mg (s) +Br2 (1) → MgB, (s) An oxidizing agent will oxdizes the opposite subsatane and features electrons. On this state, the oxidation state should be lower.Thus, oxidation state decreases from Br to 2Br Due to this fact, Br, is oxidizing agent and Mg is lowering agent.
- The Best AI Image Editing Tools in 2026: Top Picks for Creators, Marketers, and Developers - February 28, 2026
- Why More Than One Admin Is Important for Brand Pages and How to Do It Right - February 5, 2026
- What to do if you think a doctor made a serious medical error - January 30, 2026
