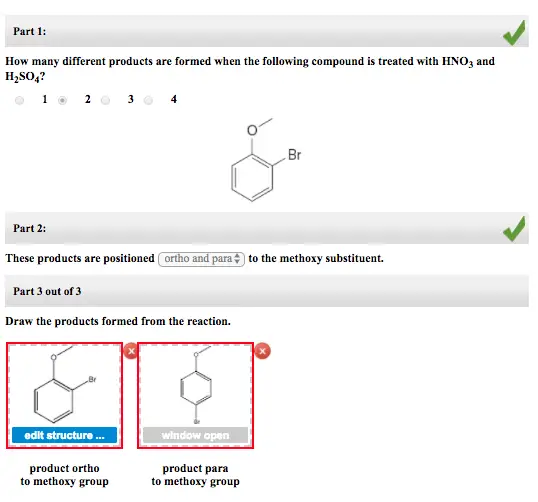
 Part 1: How many different products are formed when the following compound is treated with HNO3 and H2SO4? Br Part 2: These products are positioned ortho andpar, to the methoxy substituent. Part 3 out of 3 Draw the products formed from the reaction. Br edit structure open product ortho to methoxy group product para to methoxy group
Part 1: How many different products are formed when the following compound is treated with HNO3 and H2SO4? Br Part 2: These products are positioned ortho andpar, to the methoxy substituent. Part 3 out of 3 Draw the products formed from the reaction. Br edit structure open product ortho to methoxy group product para to methoxy group
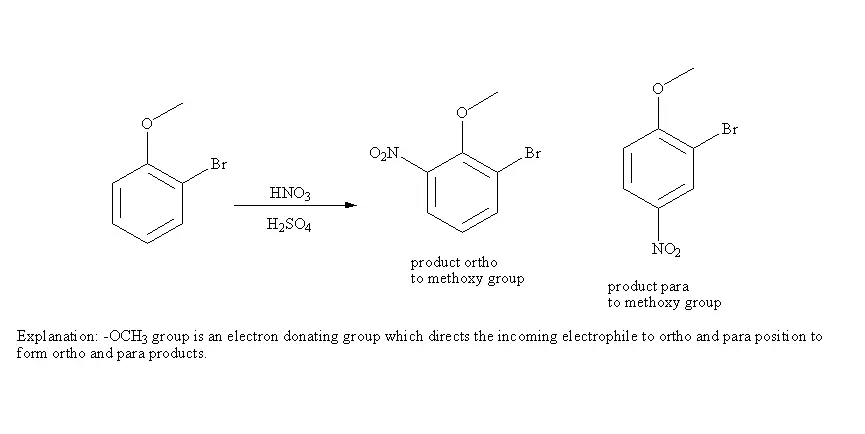
Explanation: -OCH_3 group is an electron donating group which directs the income electrophile to ortho and para position to form ortho and para products.
Latest posts by Answer Prime (see all)
- Why Mobile Networks Are Treated Differently Online - January 16, 2026
- Invisible Risks in a Connected World: The Small Things We Overlook Every Day - January 15, 2026
- What Is XDR and How It Improves Threat Detection - January 14, 2026
